Case of the Month ...
Clinical History:
A 75-year-old woman presents with epigastric pain radiating to the back. On evaluation of her MRI and CT scan there is diffuse irregularity to the entire pancreatic parenchyma raising the possibility of an autoimmune process. A follow-up endoscopic ultrasound showed a 1.3cm enhancing lesion along the dorsal/inferior margin of the pancreatic body, close to the neck. Fine needle aspiration was performed.
Diagnosis & Discussion
click on image for larger version
Figure 4 Figure 5 Figuress 1-5:
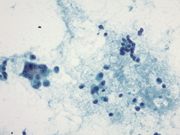
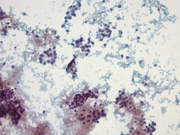
Figure 1: Pancreas, EUS-guided Fine needle aspiration. Papanicolaou stain, x200
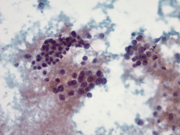
Figure 2: Pancreas, EUS-guided Fine needle aspiration. Papanicolaou stain x400
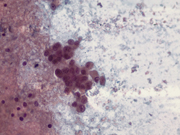
Figure 3: Pancreas, EUS-guided Fine needle aspiration. Papanicolaou stain x400
Figure 4: Pancreas, EUS-guided Fine needle aspiration. Papanicolaou stain x400
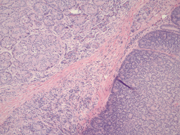
Figure 5: Pancreas, Surgical resection specimen, H&E, x10Figure 1: Two distinct populations of cells forming loose aggregates and single plasmacytoid cells. Papanicolaou stain, x200
Figure 5: Surgical resection showing cells with abundant amphophilic granular cytoplasm forming acini (top left) and smaller cells forming rosettes (bottom right), H&E, x10
Figure 2: Medium size tumor cells with moderate amount of granular cytoplasm and prominent nucleoli showing acinar formation on the left. Loose aggregate of smaller cells with inconspicuous nucleoli on the upper right. Papanicolaou stain x400
Figure 3: Medium size cells with prominent nucleoli (bottom and right) and small plasmacytoid cells (top left). Papanicolaou stain x400
Figure 4: Aggregate of tumor cells with moderate granular cytoplasm, round nuclei and prominent nucleoli. Papanicolaou stain x400Questions:
- Which of the followings is the most appropriate cytologic diagnosis?
- Solid pseudopapillary tumor
- Neuroendocrine tumor
- Acinar cell carcinoma
- Mixed acinar-neuroendocrine carcinoma
- What are the typical cytomorphologic features of this tumor?
- Monomorphic population of small plasmacytoid cells with salt and pepper chromatin
- Heterogeneous population of small plasmacytoid cells with scant cytoplasm, inconspicuous nucleoli and larger cells with granular cytoplasm and prominent nucleoli
- Clusters of cells forming papillary groups with monomorphic poorly cohesive neoplastic cells with round grooved nuclei, and indistinct nucleoli
- Cellular stroma with endocrine and acinar differentiation, heterologous bone and cartilage formation and squamoid corpuscles
- Which immunostain panel would be most helpful to confirm the diagnosis?
- Synaptophysin, chromogranin
- Trypsin, chymotrypsin
- Synaptophysin, chromogranin, trypsin, chymotrypsin
- Beta-catenin, SOX11, vimentin, CD10
- What percent of tumor cells is required to classify a pancreatic tumor as mixed acinar-endocrine carcinoma?
- Both acinar and endocrine components account for 50% of tumor cells
- Both acinar and endocrine components account for at least 25% of tumor cells
- Acinar component accounts for at least 50% while endocrine component accounts for at least 25% of tumor cells
- Endocrine component account for at least 50% while acinar component accounts for at least 25% of tumor cells
Discussion:
The smears were highly cellular showing cells arranged in three dimensional clusters, loosely cohesive groups and single cells in a necrotic background. The cell population was heterogeneous, showing small round to oval cells with moderate cytoplasm to larger cells with granular cytoplasm, nuclear enlargement and prominent nucleoli. A diagnosis of pancreatic neoplasm was rendered with a differential including acinar cell carcinoma (ACC) and pancreatic neuroendocrine tumor (PNET). Several weeks later, a distal pancreatectomy is performed. Histologic sections revealed a solid neoplasm with cells arranged in sheets, acinar structures, and trabeculae. These tumor cells showed amphophilic granular cytoplasm and prominent nucleoli. Mitotic figures were abundant with a high proliferative index shown on Ki-67 immunostain. The tumor was diffusely positive for acinar markers (trypsin and chymotrypsin). In addition, there were other foci of tumor cells arranged in rosettes where the cells were smaller and had inconspicuous nucleoli. Those tumor cells showed strong and patchy positive for endocrine markers (synaptophysin and chromogranin). Some of the tumor cells coexpressed both acinar (trypsin and chymotropsin) and endocrine markers (chromogranin and synaptophysin). The features confirmed the diagnosis of a mixed acinar-neuroendocrine carcinoma (MAEC).
MAEC of the pancreas is a rare pancreatic malignancy, with a significant expression of both acinar and endocrine markers each in more than 25% to 30% of tumor cells. Tumors containing <25% of neoplastic endocrine cells are considered to be ACC with incidental endocrine cells (1-2). The acinar component predominates (60-75%) most of the time, thus MAEC of the pancreas is considered a variant of ACC. Occasionally, the endocrine component can be predominant (1-3).
The cell origin of MAEC of the pancreas is not very clear. It has been postulated that exocrine and endocrine elements of the pancreas have a common embryologic origin. In fact, the intermixture of the two components, their cytologic similarity, and apparent coexpression of both acinar and endocrine features in some tumor cells suggest that these tumors may arise from a pancreatic stem cell with both exocrine and endocrine potential. Depending on the point at which neoplastic transformation occurs along the pathway of proliferation and differentiation of these cells, a pancreatic neoplasm may exhibit divergent differentiation. Alternatively, MAEC of the pancreas can be derived from endocrine differentiation of ACC or acinar differentiation of PNET. In some cases, a reasonable explanation is that of a “collision tumor” in which two histogenetically distinct tumors may physically grow into one another. This theory is especially attractive when the two components are restricted to different regions of the tumor (1).
MAEC affects older patients with a peak in the seventh decade and no sex predilection. Some series have shown that MAEC mostly locate in the head of the pancreas. Patients usually present with tumor mass effect without hormone excess syndrome (3). Grossly, the tumors are tan to yellow, circumscribed or encapsulated, with a soft, fleshy, or friable cut surface. Small cystic spaces, areas of hemorrhage and necrosis are occasionally present. Microscopically, the tumor is very cellular: various combinations of trabecular, acinar and glandular growth patterns are usually present. There is scant stroma with a rich plexus of capillary-sized vessels present throughout and no desmoplastic stromal response. Two different populations of neoplastic cells, one with acinar features and the other with endocrine appearance, are often recognizable by light microscopy. In some cases however, the neoplastic cells are more homogeneous and immunohistochemical studies are needed to detect the acinar and endocrine differentiation. The tumor cells of acinar component show granular cytoplasm, basally located, round to oval, uniform nuclei, and a prominent single nucleoli. The endocrine component consists of solid nests or rosettes of cells with amphophilic cytoplasm, round and small nuclei, stippled chromatin and inconspicuous nucleoli (1-4). In some series, the endocrine component was a high-grade neuroendocrine carcinoma (3). Cytologic prepaprations show small plasmacytoid cells, singly and in loose aggregates. The cells have scant delicate cytoplasm, round to oval nuclei, with mild nuclear size variation and finely granular chromatin. Some cells show increased granular cytoplasm and more prominent nucleoli (4).Immunohistochemically, these tumors exhibit evidence of acinar differentiation (trypsin, chymotrypsin, lipase, amylase and d-PAS-positive granules), and endocrine markers (chromogranin and synaptophysin); specific endocrine hormones are also present. Double immunohistochemical staining for acinar and endocrine markers reveals sharp separation of the two cell types with coexpression of both acinar and endocrine features in some cells (5).
A number of pancreatic neoplasms are usually included in the differential diagnosis with MAEC. These include pure ACC, PNET, solid pseudopapillary tumor (SPT) and pancreatoblastoma. Pure ACC and PNET cannot be reliably distinguished from MAEC without special studies. ACC is a rare pancreatic neoplasm that may contain a variable number of endocrine cells in as many as 40% of cases, so within the spectrum of pure ACC, scattered endocrine cells can be present (1). The requirement that the acinar and endocrine components each constitute a significant proportion (>25%) of MAEC is admittedly arbitrary. Similarly, preoperative fine needle aspiration helps identify whether the pancreatic mass has an endocrine component but does not correctly differentiate MAEC from pure ACC or PNET. Including MAEC of the pancreas in the cytological differential diagnosis however may be meaningful as patients with MAEC would benefit from surgery in contrast to those with high-grade neuroendocrine tumor (4).
SPT is composed of solid, cystic and pseudopapillary patterns with hyalinized fibrovascular cores. In FNA samples, SPT is characterized by high cellularity. The tumor cells form papillae lined with bland monomorphic poorly cohesive neoplastic cells with clear to eosinophilic cytoplasm, round to oval typically grooved nuclei, and indistinct nucleoli. Hyaline globules are seen. The immunophenotype shows positive staining for beta-catenin, SOX11, vimentin, alpha-1-antitrypsin, neuron-specific enolase, CD10, progesterone receptor and androgen receptor. Rosette formation and salt-and-pepper type chromatin favor PNET while ACC is characterized by loosely cohesive cell clusters, prominent nucleoli and granular, PAS-positive cytoplasm.
Pancreatoblastoma is exceedingly rare; by immunohistochemistry, the tumor shares with MAEC the presence of both endocrine and acinar differentiation. In addition, there is frequently a cellular stromal component that may even take on a neoplastic quality, with heterologous bone and cartilage formation. The presence of squamoid corpuscles, whorled nests of plump to spindled cells with keratinization is a characteristic feature of pancreatoblastoma.
The clinical behavior of MAEC tends to be aggressive, somewhat less than ductal adenocarcinoma however, suggesting that all suspected MAECs should be resected if clinically feasible (1). Metastasis is rare at presentation but recurrence and metastasis occur in most patients postoperatively. Response to chemotherapy has been poor. Nevertheless, it is reasonable to treat the high-grade component with appropriate chemotherapy (3).
In summary, MAEC are rare pancreatic neoplasms that bridge the gap histogenetically between ACC and PNET. Because of its rarity, MAEC is frequently misdiagnosed on cytology (4-5). Since there may be prognostic and therapeutic implications, MAEC of the pancreas should be kept on the differential in pancreatic FNA.
Answers:
1. D
2. B
3. C
4. BReferences:
- Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994;18(8):765-78.
- Stelow E, Shaco-Levy R, Bao F, Garcia J, Klimstra D. Pancreatic acinar cell carcinomas with prominent ductal differentiation: Mixed acinar ductal carcinoma and mixed acinar endocrine ductal carcinoma. Am J Surg Pathol. 2010;34(4):510-18.
- Ogbonna H, Garcon M, Syrigos K, Saif M. Mixed acinar-neuroendocrine carcinoma of the pancreas with neuroendocrine predominance. Case Rep Med. 2013;2013:705092.
- Sullivan P, Clebanoff J, Hirschowitz S. Hints to the diagnosis of mixed acinar-endocrine carcinoma on pancreatic fine-needle aspiration: Avoiding a potential diagnostic pitfall. Acta Cytologica 2013;57:296-302.
- Yu R, Jih L, Zhai J, Nissen NN, Colquhoun S, Wolin E, et al. Mixed acinar-endocrine carcinoma of the pancreas: new clinical and pathological features in a contemporary series. Pancreas. 2013;42(3):429-35.
Contributed by:
Rita Abi-Raad, M.D.
Assistant Professor
Department of Pathology
Yale School of Medicine
New Haven, CT, USAGuoping Cai, M.D.
Associate Professor
Department of Pathology
Yale School of Medicine
New Haven, CT, USA